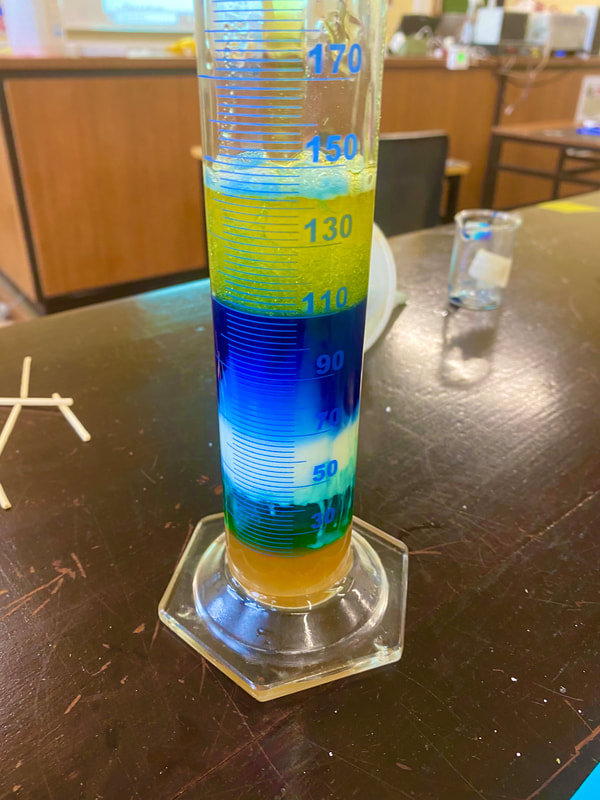
Make a Density Tower
The density of an object is calculated by dividing it's mass by it's volume.
The density of a liquid is a measure of how heavy it is for the amount measured.
In these towers, equal volumes of various liquids were measured, the liquid that weighs more is more dense.
This activity uses several types of liquids to determine which is more dense.
Lighter liquids are less dense so they float on top.
Why do some liquids weigh more than others?
Like solids, liquids are made up of different numbers of atoms and molecules.
In some liquids, these atoms and molecules are packed tightly together resulting in a denser heavier liquid e.g. syrup.
The density of a liquid is a measure of how heavy it is for the amount measured.
In these towers, equal volumes of various liquids were measured, the liquid that weighs more is more dense.
This activity uses several types of liquids to determine which is more dense.
Lighter liquids are less dense so they float on top.
Why do some liquids weigh more than others?
Like solids, liquids are made up of different numbers of atoms and molecules.
In some liquids, these atoms and molecules are packed tightly together resulting in a denser heavier liquid e.g. syrup.
Questions & Hypothesis
What liquid do you predict will have the greatest and the least density?
Will a raisin, paperclip, penny, small cork, ball of paper, and other small objects sink or float if they are placed in water, syrup and vegetable oil?
Write down what you think will happen when you place each object into the three different liquids based on your guess of the density of liquids.
These liquids will have different densities, there will be a density tower, or different layers that are visible.
Will a raisin, paperclip, penny, small cork, ball of paper, and other small objects sink or float if they are placed in water, syrup and vegetable oil?
Write down what you think will happen when you place each object into the three different liquids based on your guess of the density of liquids.
These liquids will have different densities, there will be a density tower, or different layers that are visible.
Materials
- Syrup (maple, golden)
- Milk (full fat)
- Washing up liquid
- Water
- Vegetable Oil
- Honey
- Food colouring
- Funnel
- Graduated cylinder
- Scales
- Beakers
Method
- Find the mass of the beaker using the scales.
- Pour equal amounts 30ml of each liquid into small beakers.
- Weigh the beakers again to find the mass of each liquid, record these.
- Add 2-3 drops of food colouring in water and 2-3 drops of a different food colour into the milk.
- Get your graduated cylinder to form the density tower.
- It is very important to layer all the liquids slowly in the container. Any liquid should not touch the side walls of container. You can use the funnel or back of a spoon to do that.
- Rank each liquid based on how dense you think it is. Note your predictions on the paper.
- First add the 30ml honey to the bottom of the clear graduated cylinder, being careful not to get it stuck on the sides as it is poured.
- Then slowly pour the 30ml of corn syrup on top of the honey, again being careful not to get it stuck on the side of the container.
- Then slowly pour the 30ml of maple syrup/golden syrup on top of the corn syrup, also being careful not to get any stuck on the side of the container.
- Next, add the 30ml of milk, slowly drop the milk on top of the maple syrup. Try to keep above the centre of the container for best results.
- Next is the dish soap layer.
- The next layer is coloured water. Add a few drops of food colouring to your 30ml of water and mix it together before adding this layer to the density tower.
- Next, is the vegetable oil.
- Now you will see that all the liquids are separated and they are not mixing with each other. Your density tower is ready.
Results & Discussion
Were your predictions right?
Did the raisins and other objects sink and float when you expected them to?
Did they float in one liquid and sink in another?
Why do you think they acted the way they did?
The denser a liquid is, the easier it is for an object to float on it. If one of your objects floated in the syrup but sank in the water, what does that tell you about the densities of water and syrup?
Did the raisins and other objects sink and float when you expected them to?
Did they float in one liquid and sink in another?
Why do you think they acted the way they did?
The denser a liquid is, the easier it is for an object to float on it. If one of your objects floated in the syrup but sank in the water, what does that tell you about the densities of water and syrup?