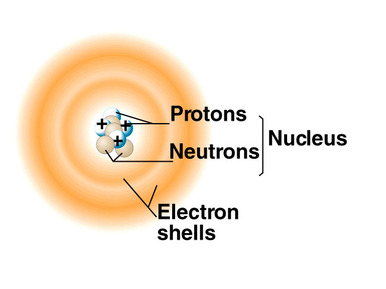
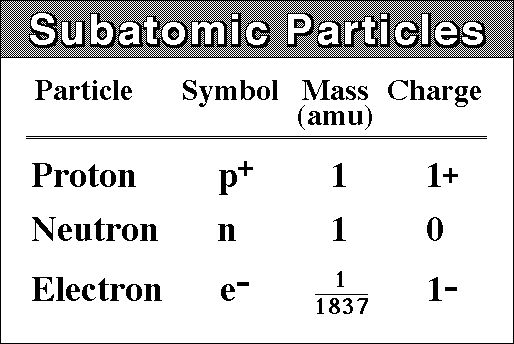
Basic atomic structure
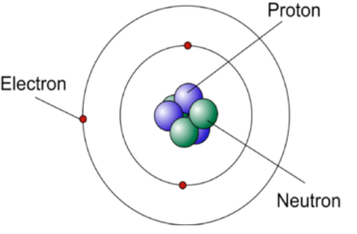 A model of a Lithium atom
A model of a Lithium atom
The Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus.
The picture on the left is a model of a Lithium atom which has the atomic number 3. This means that it has 3 protons in its nucleus and also 3 electrons in its orbits. Its Mass number is 7 which means it has 7 protons and neutrons added together. Since it has 3 protons it must therefore have 4 neutrons.
The picture on the left is a model of a Lithium atom which has the atomic number 3. This means that it has 3 protons in its nucleus and also 3 electrons in its orbits. Its Mass number is 7 which means it has 7 protons and neutrons added together. Since it has 3 protons it must therefore have 4 neutrons.
Definitions
An Atom is the smallest part of an element that has the properties of that element.
Atomic Number is the number of protons in in the nucleus of an atom.
Mass Number is the number of protons and neutrons in the nucleus of an atom.
Isotopes - the same element with different number of neutrons.
An Atom is the smallest part of an element that has the properties of that element.
Atomic Number is the number of protons in in the nucleus of an atom.
Mass Number is the number of protons and neutrons in the nucleus of an atom.
Isotopes - the same element with different number of neutrons.