Length, Time, Speed and Acceleration
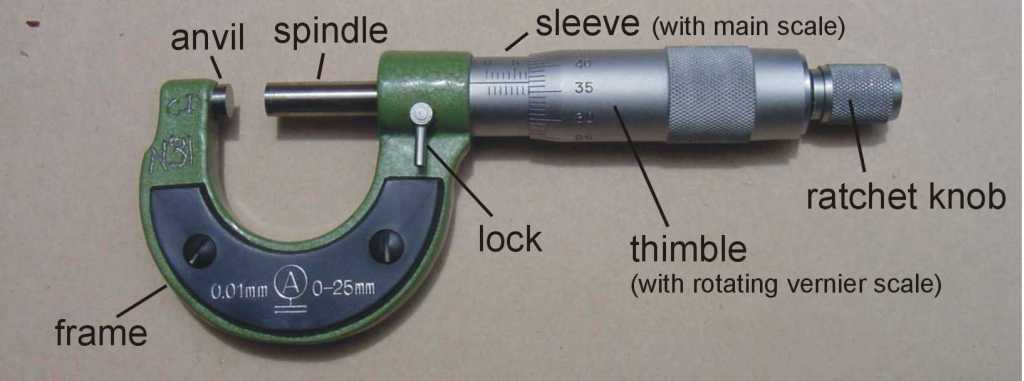
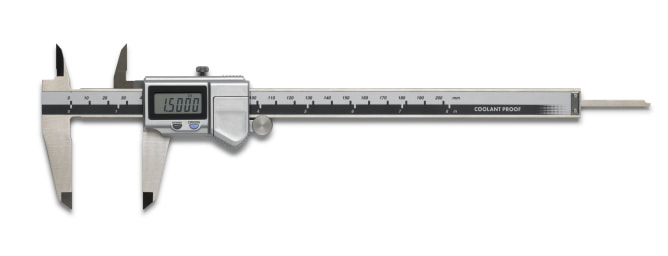
Measuring length
|
|
|
|
|
|
Area, Volume, Mass and Density
|
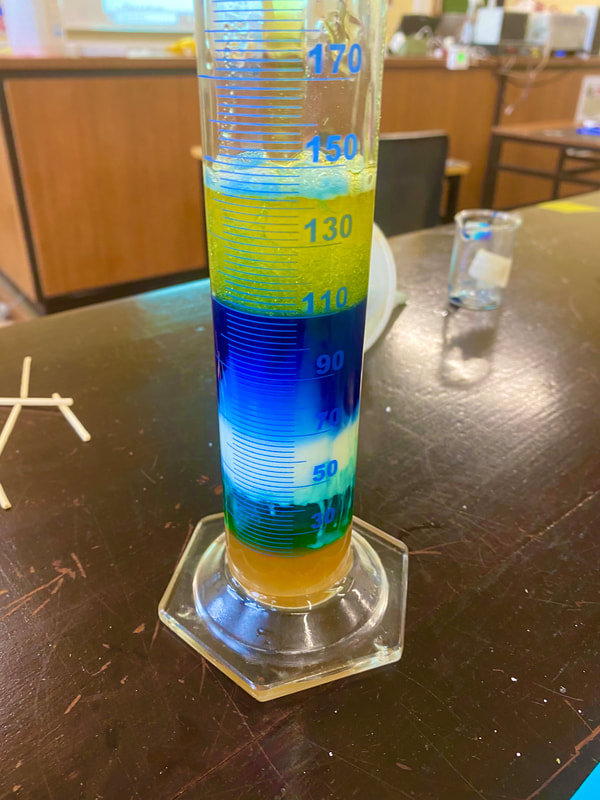
Density Tower
The density of an object is calculated by dividing it's mass by it's volume. The density of a liquid is a measure of how heavy it is for the amount measured. In these towers, equal volumes of various liquids were measured, the liquid that weighs more is more dense. This activity uses several types of liquids to determine which is more dense. Lighter liquids are less dense so they float on top. Why do some liquids weigh more than others? Like solids, liquids are made up of different numbers of atoms and molecules. In some liquids, these atoms and molecules are packed tightly together resulting in a denser heavier liquid e.g. syrup. |
|
|
|
Force
|
To the right is a video demonstrating Electrostatic Force in action!
When we rub a plastic pen up and down the sleeve of our jumper and then hold the pen near pieces of paper, the paper is attracted to the pen. This happens because the charged pen induces an opposite charge in the paper and as opposite charges attract, the paper sticks to the pen. |
|
|
|
|